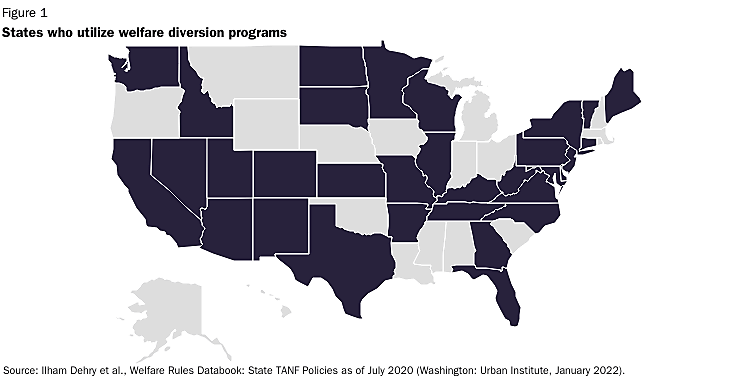
The last two weeks I wrote about the ability of states to assist welfare applicants with their immediate short-term needs while diverting them from the welfare system long-term. I also looked at the evidence that such diversion efforts had a strong record of success. Today, let’s examine why utilization of diversion options has dropped off precipitously in recent years.
Today, even in states with diversion programs available, they are seldom used. For example, in California just 18 out of 58 counties diverted even one recipient in 2018. A similar lack of utilization can be found in Georgia where in FY2021 only 12 of the state’s 159 counties used diversion even once. Statewide, just 642 individuals participated, with an average expenditure of less than $169 per participant. And, in Florida only eight families received diversion payments from October 2021 to September 2022.
One reason for this underutilization may be that an effective diversion program requires caseworkers to be trained in best practices to administer it. A 1999 study for the Department of Health and Human Services found that caseworkers were one of the most important factors in the number of applicants who are diverted. It is imperative for caseworkers to probe deeply enough into a family’s circumstances to determine if they would be benefited through diversion as it is unlikely that the applicant would even know about the program’s existence.
Complicating matters, whether to divert a family is often a matter for the caseworker’s discretion, with no hard and fast rules about who should and should not be diverted. It is not simply a question of whether the recipient’s immediate problem would be solved by a lump-sum payment, focused job search, or alternative resources; it is also about whether the diversion will successfully keep the applicant off welfare for the long term. Much of the research cited in my previous blogs suggests that those applicants most likely to remain off welfare are those with previous attachment to the labor force, job-ready skills, transportation, and childcare arrangements. Both job search and diversion to alternative resources requires even more active involvement by caseworkers who must understand the applicant’s situation and how to match them with available resources.
Responding to inquiries from Mathematica Policy Research, state officials frequently cited caseworker confusion or hesitation as a reason for not making greater use of the program. As one official told Mathematica, “Our workers like things to be black and white…this has caused us to get a lot of questions about who is eligible and who is not.”
Properly preparing caseworkers to make use of diversion programs would require states to invest up front in training and possibly in expanding the number of caseworkers. That may require short-term spending, which states are reluctant to undertake, even though it would likely result in long-term savings.
A Win-Win Proposition
Diversion programs offer a win-win opportunity for welfare reform. By keeping potential recipients off Temporary Assistance for Needy Families (TANF) and/or other welfare programs, states have the opportunity to reduce their spending over time. on these programs. At the same time Simultaneously, diversion programs can provide recipients the resources they need to escape poverty without becoming entangled in the welfare system. For many recipients, diversion should be the first option for assistance.
States that don’t yet have diversion programs should adopt one or more of these approaches. States which already have these programs (see Part I of this blog series) need to be more willing to invest in diversion. They need to train caseworkers to be more aware of diversion options, how to screen applicants, and how to craft the most effective response to the applicants’ circumstances.
At the same time, the federal government should extend the concept of non-recurrent short-term benefits to welfare programs beyond TANF and Supplemental Security Income. There is no reason why some applicants cannot be better served by diversion from most traditional welfare programs.
Not every welfare applicant will be a prime candidate for a diversion program. But, properly utilized diversion offers an opportunity for states to substantially reduce their welfare rolls (and long-term expenditures) without sacrificing their ability to assist those in need. Both state and federal governments should act to ensure wider utilization of this important tool. This is the type of welfare reform that should draw broad bipartisan support. It is both compassionate and fiscally responsible.