The Food and Drug Administration (FDA) oversees the safety of human and veterinary drugs, biological products, medical devices, foods, cosmetics, and products that emit radiation. The agency also regulates the manufacturing and marketing of tobacco.
During the COVID-19 crisis, the FDA has been more of a hindrance than a help. It put the battle weeks behind by blocking the development of private-sector virus tests, and its regulations have slowed production of hand sanitizer and facemasks.
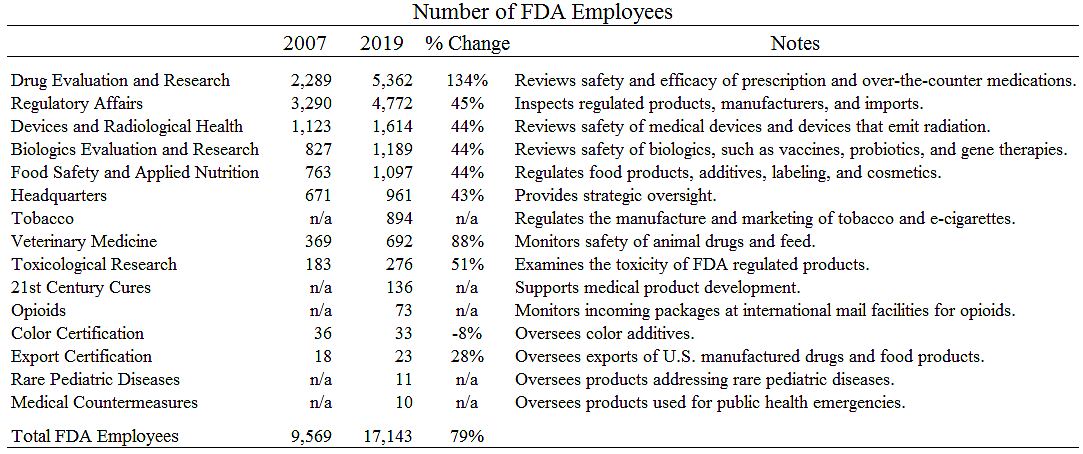
I noted the other day that FDA employment has soared since 2007. What do all the new employees do? The table below shows that the FDA bureaucracy has expanded across the board.
Total FDA employment increased 79 percent from 2007 to 2019. Part of the increase was due to new activities. For example, the FDA began regulating tobacco with the passage of a 2009 statute and now has almost 900 workers in that function.
Details about FDA’s activities are in this document. One section is titled “Fostering Competition and Innovation,” which is sadly ironic given the agency’s blunder in monopolizing federal control over COVID-19 testing.
Unfortunately, similar sorts of blunders have plagued the FDA. Henry Miller noted in Regulation that the agency’s “regulators make decisions defensively, tending to unnecessarily delay or reject new products of all sorts, from cancer drugs to vaccines and painkillers.” Drugs approved in other advanced economies have been delayed for years by the FDA, which has proved lethal in numerous cases, he notes.
Further discussions of the FDA’s pathologies are here, here, here, and here.
David Kemp assisted with this blog.