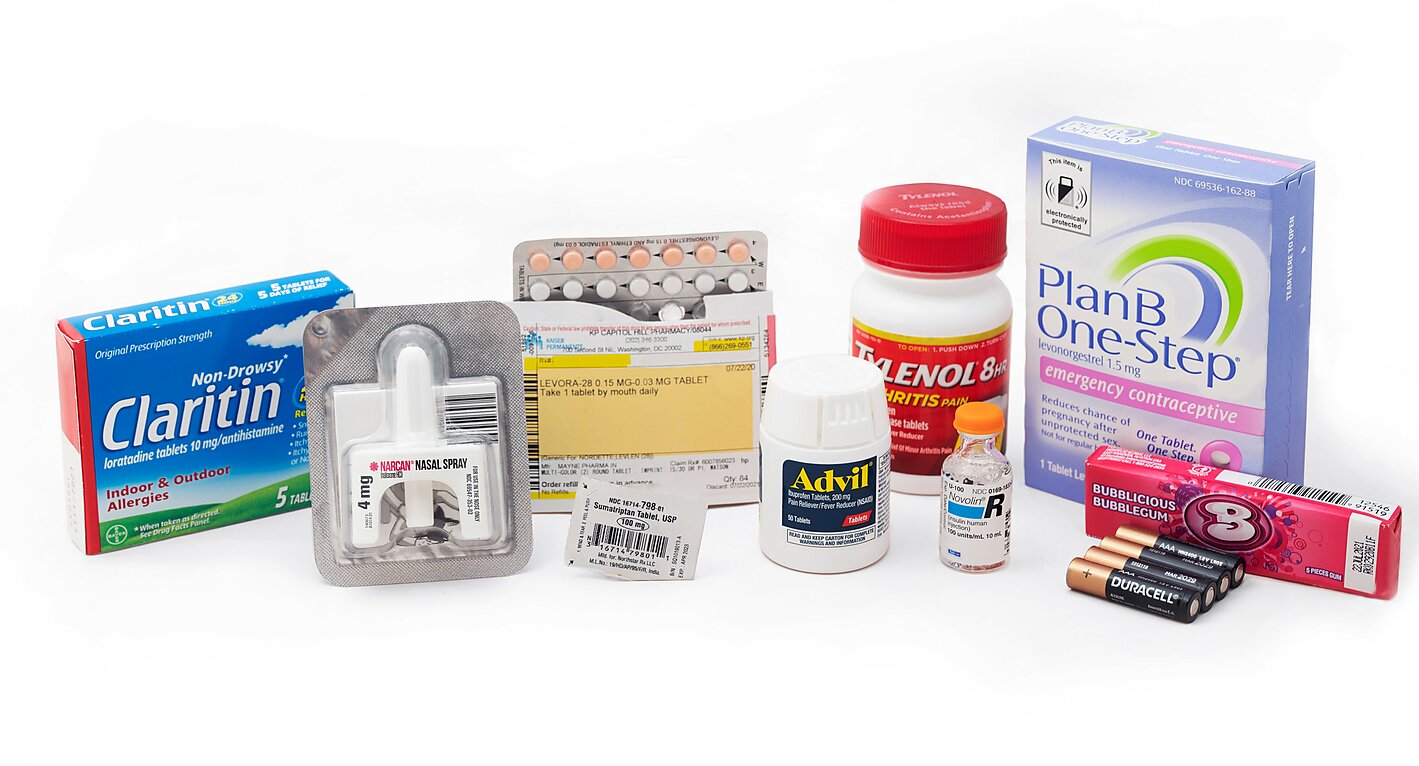
Research shows, however, that government routinely requires prescriptions for drugs that are safe for consumers to use on their own. For years, Food and Drug Administration (FDA) prescription requirements steered consumers away from safer nonsedating antihistamines toward more dangerous sedating antihistamines. More recently and for political reasons, Presidents George W. Bush and Barack Obama collectively blocked access to “Plan B” emergency contraception for more than 12 years. The FDA continues to force consumers to endure unnecessary and costly visits to their doctors before obtaining routine-use oral contraceptives and life-saving drugs such as naloxone.
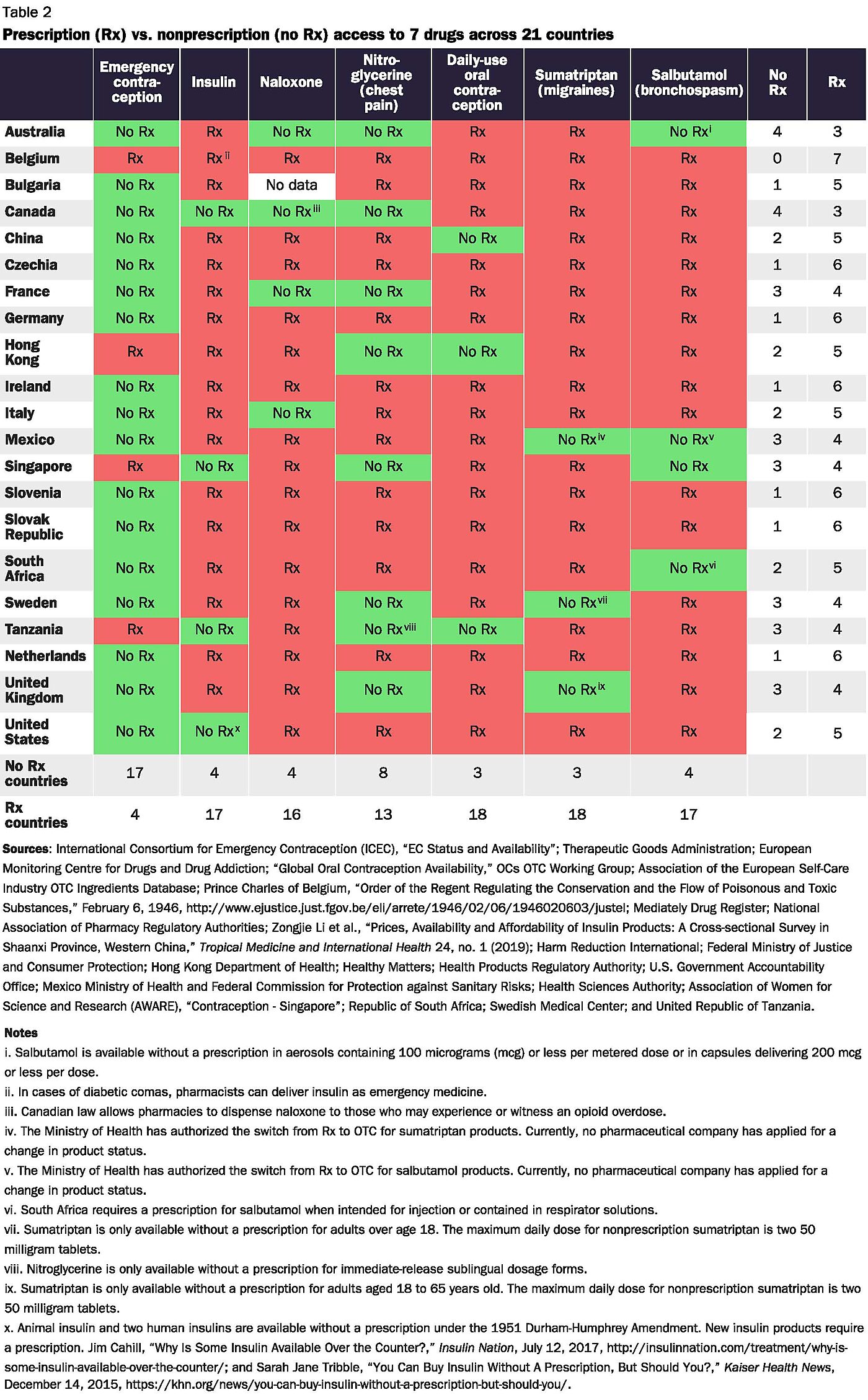
Government-imposed prescription requirements violate the rights of individuals to access the medicines they want. Vesting this power in government has left Americans with less access to medicines overall—even relative to consumers in other nations where governments also impose prescription requirements. It imposes unnecessary costs that rise during public health crises such as the COVID-19 pandemic. Evidence also suggests that government-imposed prescription requirements make patients less safe, not more.
Congress should deny the FDA any power to impose prescription requirements. Doing so would not end prescription requirements. The threat of tort liability would push pharmaceutical manufacturers to require authorization from a physician or other competent medical professional before consumers could purchase unusually dangerous drugs. Even without a statutory requirement, consumers would continue to consult health care professionals before accessing certain drugs when they see the need for expert advice. Drug manufacturers, pharmacies, and their liability insurers could develop innovative means of tailoring drug access to the risks that individual drugs pose.
Denying government the power to require prescriptions would expand drug access by reducing both drug prices and the associated nonprice costs of obtaining needed drugs. The evidence suggests that eliminating government-imposed prescription requirements would lead to more-judicious use of pharmaceuticals because consumers make more-cautious drug decisions when the choice is theirs rather than when government forces them to consult physicians. Denying the FDA this power would help ensure access to beneficial medicines during the COVID-19 pandemic and subsequent public health crises.