Policymakers can reduce overdose deaths and other harms stemming from nonmedical use of opioids and other dangerous drugs by switching to a policy of “harm reduction” strategies. Harm reduction has a success record that prohibition cannot match. It involves a range of public health options. These strategies would include medication-assisted treatment, needle-exchange programs, safe injection sites, heroin-assisted treatment, deregulation of naloxone, and the decriminalization of marijuana. Though critics have dismissed these strategies as surrendering to addiction, jurisdictions that have attempted them have found they significantly reduce overdose deaths, the spread of infectious diseases, and even the nonmedical use of dangerous drugs.
Harm Reduction: Shifting from a War on Drugs to a War on Drug-Related Deaths
The U.S. government’s current strategy of trying to restrict the supply of opioids for nonmedical uses is not working. While government efforts to reduce the supply of opioids for nonmedical use have reduced the volume of both legally manufactured prescription opioids and opioid prescriptions, deaths from opioid overdoses are nevertheless accelerating. Research shows the increase is due in part to substitution of illegal heroin for now harder-to-get prescription opioids. Attempting to reduce overdose deaths by doubling down on this approach will not produce better results.
Related Event: Dont Forget People Living in Pain: War on Opioids and Chronic Pain Patients during COVID-19
May 20, 2020 4:00 PM to 5:00 PM EDT Live Online
Social‐distancing measures exist to protect health by stopping the spread of COVID-19. But shelter‐in‐place orders, lockdowns of nonessential businesses, and prohibitions on elective medical procedures can threaten the health of patients suffering from chronic illnesses. The result is increased suffering, isolation, and despair among these Americans. Please join us for this event, where chronic pain patients, those who advocate for them, and those who treat them will discuss how current opioid policy is making the public health emergency doubly dangerous.
The Failure of Prohibition
The U.S. government’s current strategy of trying to restrict the supply of opioids for nonmedical uses is not working. The U.S. Centers for Disease Control and Prevention (CDC) reported a record-high number of opioid overdose deaths in 2015—33,091—more than half of which were from heroin.1 In 2016, the drug-overdose death rate then increased 28 percent to 42,249, with heroin and fentanyl causing the majority of those deaths, and the rate of fentanyl (plus fentanyl analog) overdoses doubling from 2015 to 2016.2 In August 2018, the preliminary estimates for 2017 were released, showing the opioid overdose rate increasing again to over 49,000, primarily due to a 37 percent increase in deaths involving fentanyl. Overdoses in 2017 from prescription drugs dropped 2 percent and overdoses from heroin dropped 4 percent.3
A study published in November 2017 finds that, while government efforts to reduce the supply of legal opioids have reduced the availability of common prescription drugs like hydrocodone and oxycodone, the use of heroin as an initiating opioid for nonmedical users has grown at an alarming rate. In 2015, more than 33 percent of heroin addicts entering treatment initiated their nonmedical opioid use with heroin, up from 8.7 percent in 2005.
Part of this effect may be economic: in 2015, the CDC director estimated the black-market price for heroin was one-fifth the price of prescription opioids.4 The gradual substitution of heroin for prescription opioids may be behind the soaring overdoses. The researchers concluded, “Given that opioid novices have limited tolerance to opioids, a slight imprecision in dosing inherent in heroin use is likely to be an important factor contributing to the growth in heroin-related overdose fatalities in recent years.”5
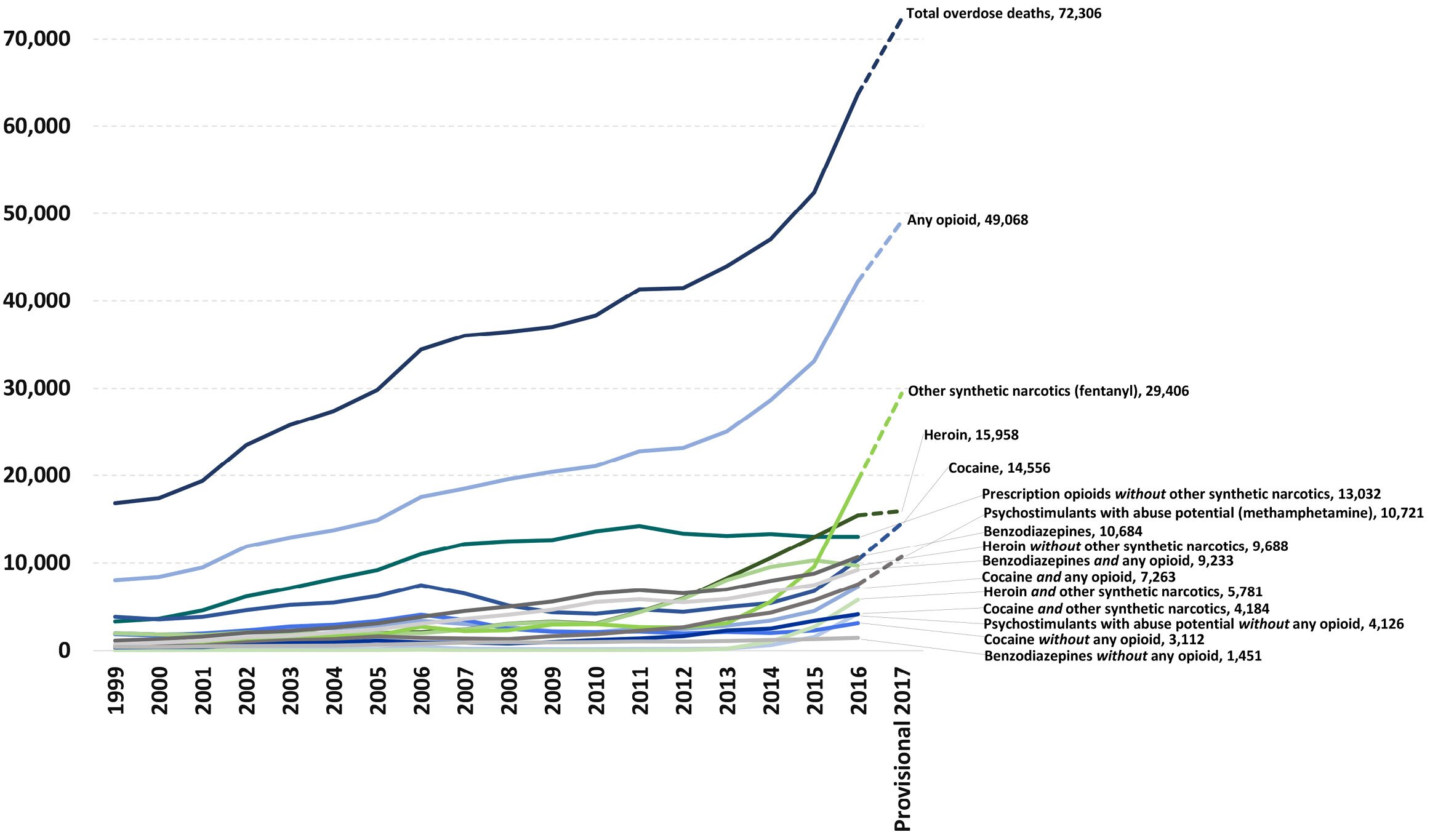
Figure 1: National overdose deaths from select prescription and illicit drugs
*Includes deaths with underlying causes of unintentional drug poisoning (x40–x44), suicide drug poisoning (x60–x64), homicide drug poisoning (x85), or drug poisoning of undetermined intent (y10–y14), as coded in the International Classification of Diseases, 10th Revision.
Sources: National Institute on Drug Abuse, Overdose Death Rates, https://www.drugabuse.gov/related-topics/trends-statistics/overdose-dea…; CDC, National Center for Health Statistics, https://www.cdc.gov/nchs/; CDC WONDER, https://wonder.cdc.gov/.
Harm Reduction
Unlike prohibition, harm-reduction strategies begin with the realistic and nonjudgmental premise that “there has never been, and will never be, a drug-free society.”6 Akin to the credo of the medical profession—“First, do no harm”—harm reduction seeks to avoid measures that exacerbate the harms the black market already inflicts on nonmedical users and to focus strictly on the goal of reducing the spread of disease and death from drug use.
Many who prefer stigmatizing rather than tolerating drug use7 criticize harm reduction as “a signal of defeat.”8 But harm reduction has a success record that prohibition cannot match. Decades of experience in several developed nations show harm-reduction strategies reduce overdose deaths, the spread of infectious diseases, and, in many cases, the nonmedical use of dangerous drugs.9
Harm reduction involves a range of public health options. These include medication-assisted treatment, needle exchange programs, safe injection sites, heroin-assisted treatment, deregulation of overdose treatments like naloxone, and decriminalization of cannabis (marijuana).
Medication-Assisted Treatment
Medication-assisted treatment provides drugs that help to wean users off opioids. Opioid-replacement therapy involves the replacement or substitution of an illegal opioid, such as heroin (diacetylmorphine or diamorphine), with a legal one that is less sedative and euphoric.10 The idea behind opioid-replacement therapy is to help the addict avoid experiencing withdrawal from heroin, reduce cravings for the drug, and eliminate the euphoria associated with heroin use. The goal is to facilitate a resumption of stability in the user’s life, end the spread of disease through needle sharing, reduce the risk of overdose and, over time, wean the user off the replacement drug. Some users stay on the replacement drug indefinitely.
Echoing other critics, in 2017 Health and Human Services secretary Tom Price characterized medication-assisted therapy as “just substituting one opioid for another, not moving the dial much.”11 The evidence tells a different story. Medication-assisted therapy decreases both exposure to infectious diseases and the risk of overdose from black-market opioids that may be laced with dangerous additives.12
The choice of opioid used in replacement therapy is a function of its absorption rate, the degree to which it binds with opioid receptors, and the duration of its effects. In some countries, such as Switzerland and Austria, orally administered slow-release morphine is occasionally used for opioid-replacement therapy. Extended-released dihydrocodeine has been used in Germany and Austria.
Methadone (brand name Dolophine) is a form of medication-assisted treatment used in the United States and many other developed countries. It has roughly the same potency as heroin, 2.5 times the strength of morphine. If injected intravenously, it will have roughly the same effect on the patient but is longer-acting than either morphine or heroin.
Also in common use is buprenorphine (brand name Subutex). Buprenorphine and methadone are administered orally. When absorbed from the intestinal tract, they bind with opioid receptors to prevent withdrawal symptoms from heroin abstinence but at absorption levels that do not lead to the sedation and euphoria that addicts experience.
A risk of buprenorphine is that users can dissolve and inject it, achieving an opioid high. However, a related medication-assisted treatment that goes by the brand name Suboxone combines buprenorphine and naloxone to create an abuse-deterrent formulation of buprenorphine. Naloxone is an opioid antagonist that attaches to opioid receptors and blocks opioid agonists (e.g., buprenorphine) from activating those receptors. Since the intestinal tract does not absorb naloxone to any significant degree, adding naloxone has little effect on patients who take the drug orally as intended. If a Suboxone recipient attempts to inject it, however, the naloxone will bind to the recipient’s opioid receptors and block the effects of the buprenorphine.
The buprenorphine in Suboxone is a partial opioid agonist, meaning it occupies some but not all of a patient’s opioid receptors. Methadone is a full agonist. It can be taken in amounts that occupy all the opioid receptors and therefore is more effective in treating patients who have grown dependent on high doses of opioids. Because buprenorphine is only a partial agonist, it causes less respiratory depression than methadone and thus has less overdose potential.
In the United States, methadone maintenance therapy started in the early 1960s. Methadone can only be dispensed at centers certified by the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA) as an Opioid Treatment Program clinic, and registered with the U.S. Drug Enforcement Administration (DEA). The patient must go to the clinic daily to receive the methadone until the treating physician deems the patient is stable enough to take the methadone at home.
The U.S. Food and Drug Administration (FDA) approved Suboxone for use as opioid replacement therapy in 2002. Subutex is no longer available in the United States. Its manufacturer took it off the market in 2011, essentially replacing it with the “abuse-deterrent formulation” Suboxone.13 Generic competitors to Suboxone, such as one selling under the brand name Zubsolv, are now available.14
Doctors may prescribe Suboxone in private clinics, as well as in community hospitals, health departments, and prisons. Doctors wishing to prescribe Suboxone as an opioid replacement must take an eight-hour class on addiction treatment (or already possess such credentials) and obtain a special license and number from the DEA. They are permitted to treat only 100 patients at a time, expandable to 275 patients after the first year, while nurse practitioners and physician assistants may only prescribe Suboxone if they obtain a waiver from SAMHSA and the DEA.15
The longer a patient stays in a treatment program, the less likely the patient will resume heroin use.16 Factors favoring retention include a higher dose of opioid replacement, free treatment, greater contacts with the clinic, and counseling.17
Retention of patients within opioid replacement therapy programs is a significant problem. Many leave the program and resume their heroin use, while some divert their methadone for intravenous nonmedical use. (Suboxone, as mentioned earlier, contains the opioid antagonist naloxone and is unsuitable for diversion.) A 2008 study in the Journal of Addictive Diseases found one-year retention rates in either methadone or buprenorphine maintenance programs averaged in the range of 50–60 percent and correlated with the doses given to patients.18 An earlier study of patients in Washington and Oregon placed retention rates even lower.19
For patients who remain in buprenorphine or methadone programs, opioid replacement therapy has been found to significantly reduce mortality from all causes of overdose. A systematic review and meta-analysis of cohort studies in the BMJ in March 2017 found methadone treatment was associated with a 69 percent reduction in all-cause mortality and buprenorphine treatment was associated with a 55 percent reduction in all-cause mortality.20
While methadone has been in use for a long time, buprenorphine (Suboxone) has been less widely used and for a shorter period, so there are few good studies comparing the two to determine which is the better treatment. Cochrane literature reviews are highly regarded for their quality and rigor, and Cochrane officially collaborates with the World Health Organization. A 2003 Cochrane review found buprenorphine considerably less successful than methadone in retaining patients in treatment.21 A 2012 review found methadone to be slightly more successful and less expensive than buprenorphine as an opioid replacement.22 However, a 2015 study by Peddicord et al. concluded that “the research does not indicate that one medication is a better option than the other. This decision must be made on an individual basis after reviewing important patient factors such as health status and access to the medication.”23
A different approach to medication-assisted therapy is naltrexone (Vivitrol). Naltrexone is a long-acting opioid antagonist that blocks the opioid receptors, similar to naloxone. Thus, it may precipitate withdrawal symptoms in patients who are physically dependent on opioids. It can be taken orally, with the effects lasting 24 to 48 hours, or injected intramuscularly in an extended-release form on a monthly basis. For it to be effective, treatment should start only after the patient has detoxified. The rationale behind naltrexone treatment is to provide negative feedback to the use of opioids, following detoxification, when the patient is exposed to the usual social cues and stressors that would lead an addict to resume use of the drug. The hope is that by blocking the opioid, naltrexone will eventually eliminate the patient’s conditioned response of turning to opioids in such situations. Subdermal naltrexone implants that slowly release naltrexone have received government approval for use as an adjunct to the oral therapy.
A 2011 Cochrane analysis showed that oral naltrexone therapy, because of its short duration of action, had high drop-out rates and was no better than placebo, with or without adjuvant psychotherapy.24 The extended-release form of naltrexone presumably would yield better results, but there are very few studies on that approach thus far. A few studies have shown improved retention rates (53–70 percent) when using the intramuscular or subdermal/oral approach.25
Medication-assisted treatment is already an accepted approach in the United States and deserves further support and development. Congress should reduce or eliminate the complex application processes and tight restrictions it imposes on health care practitioners who provide medication-assisted treatment. It should allow practitioners to take on more patients and reduce administrative hurdles that inhibit participation in such programs. It should eliminate requirements that nurse practitioners and physician assistants must obtain special waivers from SAMHSA and the DEA to provide these services. It should liberalize restrictions on methadone maintenance programs to allow the creation of more centers, particularly in hard-hit communities. It should allow primary care practitioners with an interest in treating substance abuse disorders to prescribe methadone to their patients in an ambulatory setting, as they may now do with Suboxone. This policy has been successful for decades in several developed countries, such as Australia, the United Kingdom, and Canada.26 Until Congress acts, SAMHSA and the DEA should themselves take as many of these steps as is consistent with the law.
Needle Exchange Programs
Needle exchange programs seek to reduce the spread of HIV, hepatitis, and other infectious diseases by providing clean needles and syringes for users of heroin and other injectable drugs.
The Netherlands developed needle exchange centers in the 1970s in response to an outbreak of hepatitis B. The idea gained acceptance in other countries with the advent of the AIDS pandemic. The oldest continuing needle exchange program in the United States, located in Tacoma, Washington, has been operational since 1988.27 As of 2012, needle exchange programs operated in at least 35 states.28 Congress banned federal funding of needle exchange programs in 1988 and then lifted the ban in 2009.
Needle exchange centers are often in clinics that offer referral for addiction therapy and counseling. To increase outreach, some programs operate mobile vans or delivery services, or else have centers along pedestrian routes.29 Many offer HIV and hepatitis testing, male and female condoms, and bleach and alcohol to clean drug paraphernalia.
Needle exchange programs appear to reduce the spread of infectious disease. Seven federally funded studies conducted between 1991 and 1997 found needle exchange programs reduce the risk of HIV infections among intravenous drug users and their partners.30 A 2013 systematic review conducted by the CDC confirmed that needle exchange programs are associated with a decreased prevalence of HIV and hepatitis C infections.31 A 2014 systematic review and meta-analysis of 12 studies comprising 12,000 person-years found that needle exchange programs coincide with a 34 percent reduction in the rate of HIV transmission, with a 58 percent reduction among the six studies that were of a “higher quality.”32 SAMHSA maintains a bibliography of studies on needle exchange programs on its website, and endorses needle exchange programs for their “efficacy and facilitating entry into treatment for intravenous drug users (IDUs) and thereby reducing illicit drug use.”33 The CDC endorses and promotes the implementation of needle exchange programs with guidance and, in some cases, financial assistance to local jurisdictions.34
Many state and local laws inhibit needle exchange programs.35 Some states outlaw the sale or even the possession of syringes or needles without a prescription.36 In a 2009 national survey, a significant number of needle exchange programs reported that police confiscate syringes and even arrest clients on their way to and from needle exchange centers. Reports of confiscation and arrest were more than four times more prevalent around needle exchange programs serving areas where clients were predominantly people of color.37
Safe Injection Sites
While needle exchange programs seek to decrease the spread of infectious diseases, safe injection site programs have more ambitious goals.38 Safe injection sites allow intravenous drug users to inject in a clean and safe environment, with almost no chance of overdose death, free from harassment as well as the risks of theft and physical or sexual assault. Safe injection sites furnish sterile syringes and needles as well as a clean, clinical setting where intravenous drug users can inject illicitly obtained substances. Onsite health care professionals have naloxone available to treat overdoses and can refer patients for medical treatment and rehabilitation. Like needle exchange programs, safe injection sites also prevent the patient from passing used needles and syringes to others.
As of 2016, about 100 safe injection sites operated in 66 cities around the world.39 The first professionally staffed injection room opened in Rotterdam, the Netherlands, in the early 1970s. The Dutch government officially sanctioned such centers in 1996.40 In 1986, a safe injection site that started informally in a café in Bern, Switzerland, eventually received government sanction for users over the age of 18. During the 1990s and early 2000s, legal facilities opened in Switzerland, Germany, the Netherlands, Spain, Luxembourg, Norway, Canada, and Australia.41 Germany’s first “drug consumption room” (DCR) opened in Berlin in 1994. Australia opened its first facility in the Kings Cross district of Sydney in 2001. Canada’s first facility, called “Insite,” opened in the Downtown Eastside district of Vancouver in 2003.
The evidence is strong that safe injection sites reduce the transmission of HIV and hepatitis, prevent overdose deaths, reduce public injections, reduce the volume of shared or discarded syringes, and increase the number of drug users entering treatment programs.42 A 1996 report on “injecting rooms” in Switzerland concluded:
Injecting rooms have enabled the adoption of less hazardous injecting practices, reduced the number of overdose deaths, minimised the nuisance to the community of injecting in public places and probably reduced HIV transmission. The Centres are well-tolerated in Swiss communities. Some [intravenous drug users] have entered treatment as a result of attending injecting rooms.43
The Canadian Medical Association Journal reported, “Twelve weeks after Insite opened in September 2003 … the average daily number of drug users injecting in public dropped by nearly half while the average daily number of publicly discarded syringes and injection-related litter also fell significantly.”44 In 2010, the British Columbia Center for Excellence in HIV/AIDS summarized the research on the effects of Insite on “the public order and public health.” It reported Insite “reduced HIV risk behavior” (e.g., sharing needles), promoted addiction treatment, provided “a safe space away from the dangers of the street-based drug scene,” and “reduce[d] the risk of violence against women, particularly violence that occurs before or during the injection process.”45
A 2011 retrospective analysis of the 25 DCRs then operating in Germany summarized:
- “DCRs make a decisive contribution for survival assistance and risk minimization when consuming illegalized drugs.
- “DCRs provide a bridge function towards further medical and psycho-social support with their low-threshold and acceptance-oriented contact opportunities.
- “DCRs make a significant contribution towards the reduction of problems related to the open drug scene in the cities.
- “DCRs significantly contribute to limiting the spread of infectious diseases such as hepatitis and HIV in addition to individual health protection.”46
A 2011 paper found a dramatic decrease in overdose deaths in communities in Vancouver and Sydney served by these programs, areas with populations that typically are at higher risk of HIV and hepatitis transmission.47 Another 2011 study found overdoses within the community dropped dramatically after the opening of the Vancouver site.48 Positive outcomes from the safe injection site in Sydney, Australia, have led to calls, endorsed by the Australian Medical Association, to expand the program throughout the country.49
Despite worldwide success with safe injection sites, and although needle exchange programs have proliferated in the United States with the encouragement of the CDC,50 no legal safe injection sites currently exist in this country. Seattle announced plans to establish the first safe injection site in the United States in 2016,51 but significant opposition has delayed its opening.52 In August 2017, San Francisco announced the creation of a task force to explore establishing one,53 but no site had opened at the time this report was written. Even so, one safe injection site has been operating underground in the United States since 2014 according to one popular54 and one academic55 article. Because of potential legal issues, the authors declined to identify its location. According to a study in the American Journal of Preventive Medicine, the underground site has made possible the onsite reversal of four overdoses and has seen no deaths and no problems with community acceptance.
Critics view safe injection sites as flouting the law, express discomfort with what they see as government sanctioning of intravenous drug use and other illegal activities, and argue that safe injection sites do little to deter illegal drug use.56 These concerns are understandable, but the evidence shows safe injection sites save lives by reducing overdose deaths and have likely saved lives by reducing the spread of deadly diseases and violence against drug users.
Heroin-Assisted Treatment
Despite the successes of needle exchange programs and safe injection sites, patients who use these facilities are still injecting substances they obtained on the black market and whose purity, quality, and dosage are unknown. Illicit-heroin suppliers increasingly lace their products with fentanyl, which increases the intensity of the drug but also increases the risk of overdose.57 In some cases, suppliers lace illicit heroin with carfentanil, colloquially referred to as “elephant tranquilizer,” which is 30 to 50 times more powerful than fentanyl.58
Heroin-assisted treatment—in effect, supplying patients with controlled doses of heroin—eliminates uncertainty about the purity, quality, and dose of street heroin and more potent opioids. It also mitigates the patient retention problem seen with medication-assisted treatment, particularly opioid-replacement therapy.59 Critics of heroin-assisted treatment worry that it creates the perception that intravenous heroin use can be safe and that it encourages drug use among people who would otherwise be deterred. While heroin is inherently risky, the evidence shows heroin-assisted treatment reduces both the risks and incidence of heroin use.
The United Kingdom began using heroin-assisted treatment in a limited fashion as early as the 1920s with some anecdotal successes. However, the country began to taper off its use in the 1970s in cooperation with the U.S.-led war on drugs.60 More recent experience has encouraged several countries to adopt heroin-assisted treatment into their national health systems. In 1994, in the face of one of the largest open drug scenes in Europe, Switzerland began large-scale trials of such therapy. Policymakers considered it such a success that they made the program permanent. The strategy primarily targeted intravenous drug users for whom methadone maintenance was unsuccessful, either because the patients dropped out of the program or because they continued to use intravenous heroin, sometimes in addition to the methadone. Patients accepted into the program had to be at least 18 years of age and were required to surrender their driver’s license. To qualify for inclusion, they had to have been addicted daily for at least two years and to have had two or more failed attempts at more conventional methods of therapy such as methadone maintenance or other medication-assisted treatment. Pharmaceutical-grade heroin (diamorphine) can only be obtained at the clinic. Patients may receive up to three doses per day. The majority (68 percent) receive the heroin by injection, but some take it in pill or liquid form. If patients have been in the program for at least six months and can hold a job, they may be allowed to take heroin home in pill form to use away from the clinic.
The results were impressive and persuasive. In 2006, Swiss investigators reported in The Lancet, “The population of problematic heroin users declined by 4 percent a year” and “the harm-reduction policy of Switzerland and its emphasis on the medicalisation of the heroin problem seems to have contributed to the image of heroin as unattractive for young people.”61 A 2011 Cochrane analysis comparing heroin-assisted treatment to more commonly used opioid-replacement regimens corroborates these findings.62 An analysis of the Swiss program’s results from 1994 to 2017 found much greater patient retention than in other forms of opioid-replacement therapy. The average length of time patients remain in the program is three years. Some stay indefinitely: 20 percent of the original patients were still in the program at the time of the study. Felony crimes by patients fell 60 percent. The incidence of patients selling heroin—many heroin addicts sell heroin in order to support their drug habit—fell by 82 percent, leading to a reduction in street sales of heroin. The reduction in the street use of heroin also reduced the exposure to heroin for teens experimenting with drugs. No overdose deaths have been reported since the program’s inception. Swiss health authorities have noted a significant drop in new hepatitis and HIV infections. They also reported that patients had “improved social functioning” (e.g., stable housing and reduced unemployment).63 In 2008, a referendum to make the program a permanent legal part of the Swiss health system passed with 68 percent of the vote.64
The success of Switzerland’s program led to trials in Germany and the Netherlands, after which each began providing heroin-assisted treatment through their health systems in 2008. The results in Germany65 and the Netherlands66 are comparable to those in Switzerland. A comprehensive study of the German program published in 2008 reported that 40 percent of all patients found employment after four years in the program.67
Spain began a trial program in Andalusia in 2006. Belgium is considering adopting heroin-assisted treatment as part of its national health system. Canada began trials in Vancouver and Montreal in 2009.68 The United Kingdom expanded its program in 2009.69 Each program is slightly different, but all operate under essentially the same principles. In the Netherlands, for example, patients can inject diamorphine twice a day and are given a take-home dose of oral methadone for the evening.
In 2012, the European Monitoring Centre for Drugs and Addiction reviewed randomized clinical trials of heroin-assisted treatment programs in Switzerland, Germany, the Netherlands, Spain, the United Kingdom, and Canada, involving a total of more than 1,500 patients, comparing the results with methadone maintenance therapy for long-term refractory heroin-dependent patients. The Centre concluded:
Across the trials, major reductions in the continued use of “street” heroin occurred in those receiving SIH [supervised injectable heroin] compared with control groups (most often receiving active Methadone Maintenance Treatment). These reductions occasionally included complete cessation of “street” heroin use, although more frequently there was continued but reduced irregular use of “street” heroin, at least through the trial period (ranging from 6 to 12 months). Reductions also occurred, but to a lesser extent, with the use of a range of other drugs, such as cocaine and alcohol. However, the difference between reductions in the SIH group and the various control groups was not as great (compared with major reductions in the use of “street” heroin).70
In 2009, Canadian investigators reported in the New England Journal of Medicine the results of a randomized controlled study of 111 patients comparing methadone to heroin for the medication-assisted treatment of addiction:
Methadone, provided according to best-practice guidelines, should remain the treatment of choice for the majority of patients. However, there will continue to be a subgroup of patients who will not benefit even from optimized methadone maintenance. Prescribed, supervised use of diacetylmorphine appears to be a safe and effective adjunctive treatment for this severely affected population of patients who would otherwise remain outside the health care system.71
A 2011 Canadian study noted greater client satisfaction and retention with heroin-assisted treatment than methadone maintenance.72 A 2012 study in the Canadian Medical Journal found heroin-assisted treatment superior to an enhanced methadone maintenance program and more cost-effective in the long run, primarily because heroin-assisted treatment tends to retain patients in the program.73
Heroin-assisted treatment has proved effective as a harm-reduction modality, particularly in patients who have failed other forms of opioid-replacement therapy. In addition to improving the retention of resistant patients, it reduces the sale and street presence of intravenous heroin, reduces crime, and may reduce teen experimentation with the drug.
Heroin is currently classified by the FDA as a Schedule I drug, under the authority of the Controlled Substances Act of 1970. Schedule I drugs are deemed to have no accepted medical use, lack safety even under medical supervision, and have a high potential for abuse. Consequently, the drug is illegal. But heroin (diacetylmorphine or diamorphine) is indeed used medically throughout the developed world, and opioids with greater potency and safety concerns are legally used in U.S. medical practice.74 The DEA should reschedule diacetylmorphine, and the FDA should approve clinical trials in heroin-assisted treatment.
Relaxing Restrictions on Naloxone
Removing government restrictions on naloxone, a drug that can save the lives of users who overdose on heroin, is among the least controversial harm-reduction measures. The CDC has recommended making the drug more widely available since 2013.75 The FDA has likewise voiced support.76
Naloxone (Narcan) was developed in 1961 and approved for use in the United States for the treatment of opioid overdose in 1971. It binds to opioid receptors and displaces opioids already bound to those receptors. It can therefore reverse the respiratory depression caused by an opioid overdose within 2 to 8 minutes. Its effects last about 30 to 60 minutes. The quickest route of administration is intravenous. Other routes are intramuscular or via nasal spray. Naloxone is very poorly absorbed from the intestinal tract.
Naloxone has few to no side effects if opioids are not present in the patient. In an opioid-dependent user, however, it can precipitate withdrawal symptoms (by displacing the opioid molecules already bound to the patient’s receptors). Naloxone is nevertheless so effective at reducing deaths from overdose that the World Health Organization includes the drug on its “list of essential medicines” for the treatment of opioid dependence.77 Naloxone is a prescription drug but not a controlled substance because it has no abuse potential.
State governments impose various restrictions on naloxone. Several states prohibit third-party prescriptions (i.e., the prescription of a medication for someone other than the person for whom it is intended). Such laws make it difficult to administer naloxone to overdose victims.
At the urging of the U.S. Conference of Mayors, the American Medical Association, the National Association of Boards of Pharmacy, and other organizations, all 50 states have made modifications in their laws to promote the availability of naloxone. Jurisdictions across the United States are increasingly equipping first responders (police, firefighters, and the like) with naloxone.78 A 2015 meta-analysis found that providing naloxone even to untrained bystanders significantly reduces overdose deaths.79 All 50 states and the District of Columbia have thus passed laws making it easier for lay people and other third parties to access naloxone.80 In many cases, to comply with the FDA requirement that prescription drugs must be prescribed by a health care provider licensed by the state, a pharmacist can prescribe the drug.81 Nevertheless, many people who live with or are otherwise close to opioid abusers still remain hesitant to divulge such information to pharmacists. To address this issue, many states also designate harm-reduction facilities and other nonprofit organizations as distributers of naloxone. Even so, the threat of arrest and prosecution deters many bystanders from calling first responders to the scene of an overdose, leading to otherwise preventable deaths.
Forty states and the District of Columbia have mitigated this problem by passing “Good Samaritan” laws that provide immunity to people who in good faith report an overdose to a first responder. The laws vary by state. In some states, a person who calls for an ambulance to save an overdose victim is still subject to arrest if found in possession of an illicit drug or drug paraphernalia. Some laws allow the reporting of the overdose to mitigate the sentencing of the arrested reporter.82 A University of Washington survey in 2011 found that 88 percent of people who use drugs would be more likely to call emergency responders during an overdose with a Good Samaritan law in place.83 A 2017 study of naloxone access laws from 1999 to 2014 found a reduction in opioid-related deaths ranging from 9 percent to 11 percent with no increase in the nonmedical use of opioids.84 However, it found no statistically significant effect of Good Samaritan laws on opioid-related deaths and little evidence that they increase nonmedical opioid use. All states should implement and expand such protections for those who report overdoses to first responders.
Even with such measures in place, there will still be many opioid-dependent patients and third-party contacts who are reluctant to reveal themselves to pharmacists or other legally designated dispensers of naloxone for fear of eventual intervention by law enforcement. Policymakers can solve that problem by making naloxone—a drug with a proven record of safety85—available over the counter.86
Relaxing Restrictions on Cannabis
While cannabis traditionally has not been considered part of the harm-reduction armamentarium, its potential for ameliorating opioid abuse and overdoses deserves attention. The widespread legalization of cannabis (marijuana) for medicinal and recreational use has the potential to reduce opioid abuse and related harms, including overdose and death.
To date, 21 states have legalized cannabis for medicinal purposes. Eight states and the District of Columbia have legalized it for recreational use.87 A 2014 study from the Johns Hopkins School of Public Health examined medical cannabis laws and state-level death certificates from all 50 states from 1999 to 2010 and found, “The yearly rate of opioid painkiller overdose deaths in states with medical marijuana laws … was about 25 percent lower, on average, than the rate in states without these laws.”88 A 2018 study by the RAND Corporation found that states permitting medical marijuana dispensaries saw decreased rates of opioid addiction and overdose.89 Researchers at the University of Michigan School of Public Health reported in 2016 that chronic pain patients who used medical cannabis reduced their use of opioids by 64 percent.90 A June 2017 University of California, Berkeley study reported that medical cannabis enabled 97 percent of chronic pain patients to decrease the amount of opioids they were taking, and that 81 percent found cannabis alone more effective than cannabis and opioids in combination.91 A 2018 study of Medicare Part D patients by researchers at the University of Georgia found a decreased rate of opioid use for the control of pain in states where medical cannabis was legally available.92 A 2018 report from the University of Kentucky on a study of all Medicaid fee-for-service and managed care patients across the United States from 2011 to 2016 found a decrease in opioid prescribing in states where medical marijuana was legally available, with an even greater reduction in states where both medical and recreational marijuana were available.93
Theories vary as to why legal cannabis correlates with decreased opioid abuse and overdose rates. Both recreational drug users and chronic pain patients may find cannabis more readily available, more tolerable, and safer. The question deserves further study.
Congress should legalize cannabis production, distribution, and consumption, while states should continue legalizing the substance for both medicinal and recreational use. The evidence suggests that, among other benefits, a bonus effect of legalization may be a decrease in opioid use, dependence, and overdose deaths. While opponents of legal cannabis have long warned the substance could be a “gateway” to more psychoactive drugs, cannabis may instead be an “off-ramp” drug for those who might otherwise take opioids for nonmedical purposes.
Cost-Effectiveness of Harm Reduction
Harm reduction strategies reduce the spread of diseases such as HIV and hepatitis. They also reduce the risk of overdose. While these strategies require public expenditures, on balance those costs are less than the public health, law enforcement, and incarceration costs incurred under the current approach to substance use and abuse.
A 2015 review by researchers at the Kirby Institute in Australia found the overall unit cost of harm reduction programs is low but varies depending on the method employed. The authors reviewed studies and systematic reviews from various regions. Needle exchange programs were found to be the least expensive form of harm reduction, costing $23 to $71 per intravenous drug user per year. One study indicated that needle exchange programs “are cost saving when compared to the lifetime costs of HIV/AIDS antiretroviral treatment,” while another “estimated that not only did [needle exchange programs] reduce the incidence of HIV by up to 74 percent over a 10-year period in Australia, but found that they were cost-saving and had a return on investment of between $1.3 and $5.5 for every $1 invested.” Based on evidence of effectiveness and low cost, the researchers considered needle exchange programs “one of the most cost-effective public health interventions ever funded.” Medication-assisted treatment was more expensive, but those costs were far outweighed by the larger benefits that result from a reduction in the number of relapses of substance abuse as well as lower rates of criminal activity and incarceration for drug-related crimes. The researchers concluded that harm reduction programs, particularly comprehensive strategies that include multiple modalities, were a “good value for the money invested.”94
A study of an unsanctioned supervised injection facility in Vancouver, British Columbia, concluded that the facility is highly cost-effective and reduces the transmission of deadly diseases:
A conservative estimate indicates that the SIF location that provided assisted injections has a benefit-cost ratio of 33.1:1 due to its low operational cost. At the baseline sharing rate, the facility, on an average, reduced 81 HCV and 30 HIV cases among PWID [people who inject drugs] each year. Such reductions in blood borne infections among PWID resulted in annual savings worth CAN$4.3 million dollars in health care expenditure.95
The study did not examine whether the presence of staff equipped with naloxone generated any savings attributable to a reduction in emergency overdose calls.
A World Bank Group working paper found needle exchange and medication-assisted treatment programs in Malaysia to be cost-effective as well and are “expected to produce net cost-savings to the government in the future.”96
A 2017 white paper by the West Virginia Department of Health and Human Resources Bureau for Public Health cited studies estimating that 15 to 33 percent of HIV cases could be averted through needle exchange programs, with a cost savings of between $20,947 and $34,278 per HIV case averted. Much of these costs are borne by the state’s Medicaid program.97
Conclusion
Ninety-three years after Congress banned the manufacture, distribution, sale, and possession of heroin, and 48 years after President Richard Nixon declared a “war on drugs,” drug prohibition has proved a failure. People are dying largely because of drug prohibition. Evidence continues to mount that curtailing prescription opioid availability only serves to drive nonmedical users to heroin, with increasing numbers of nonmedical users initiating their opioid abuse with that substance.98 When drug users obtain opioids on the underground market, they cannot be certain as to the purity, sterility, or dose of the product, let alone whether the substance is laced with a more dangerous and potent opioid such as fentanyl. Fear of harassment by law enforcement deters illegal users from availing themselves of clean needle exchange programs. Fear of arrest discourages them from calling first responders when they witness an overdose on the street. Many drug users also become dealers in the illicit market to support their habit, helping to perpetuate and exacerbate the problem. Efforts to reduce opioid abuse have not reduced overdose deaths and may have caused them to rise. Federal and state governments should end drug prohibition.
The current approach of trying to reduce opioid overdoses by limiting the supply of prescription opioids is based on the incorrect assumption that most opioid abusers and addicts begin as patients who become addicted after receiving prescription opioids by health care practitioners in order to treat their pain.99 The evidence increasingly shows that most opioid abusers initiate drug use for nonmedical reasons.100 Though these efforts have succeeded in reducing the number of opioids manufactured and prescribed, that is of little benefit since overdose death rates continue to climb. These findings strengthen the case for viewing opioid abuse as a psychosocial challenge rather than a product of the way health care practitioners treat pain.101 By misdiagnosing the opioid crisis, policymakers both exacerbate the crisis and cause many chronic pain patients to suffer needlessly.
Narcotics prescription data banks and continuing medical education programs on the rational use of opioids and other narcotics can help health care practitioners who treat patients in pain. But efforts to limit the supply of opioids or opioid prescriptions curtail the justifiable use of opioid analgesics, intrude on the doctor-patient relationship, and lead many physicians to practice in fear. Worse, it may be driving desperate pain patients to the illegal market, with all the risks that entails.102 There have been numerous reports of patients whose desperation drove them to suicide.103 One North Carolina internist and geriatrician maintains a growing list of chronic pain patients who have resorted to suicide after being cut off from their opioid medications.104
Short of ending the war on drugs, policymakers should convert it into a war on drug-related deaths by redirecting resources to programs focused on harm reduction. Needle exchange programs reduce the risk and spread of communicable and infectious diseases and provide addicts opportunities to enter rehab programs. Safe injection sites provide an environment free from harassment, theft, and assault, with health professionals standing by to treat overdoses with naloxone, to discard syringes after use, and to encourage enrollment in drug rehab programs. Heroin-assisted treatment provides a safer alternative to those for whom other medication-assisted therapy has proven ineffective and reduces the illicit-heroin trade. Deregulating naloxone can empower an addict’s loved ones and other third parties to save lives. Legalizing medicinal and recreational cannabis can reduce opioid use and overdoses. When it comes to harm reduction, the evidence does not point to one clear, best method. Policymakers should pursue an “all of the above” strategy.
Notes
1 CDC, “Opioid Overdose,” last updated October 19, 2018, https://www.cdc.gov/drugoverdose/index.html.
2 Holly Hedegaard et al., “Drug Overdose Deaths in the United States, 1999–2016,” National Center for Health Statistics Data Brief no. 294 (December 2017), https://www.cdc.gov/nchs/products/databriefs/db294.htm; and National Institute on Drug Abuse, “Overdose Death Rates,” August 2018, https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
3 Jacob Sullum, “New CDC Numbers Show the Drug War Continues to Make Opioids Deadlier,” Reason, August 15, 2018, http://reason.com/blog/2018/08/15/new-cdc-numbers-show-the-drug-war-contin.
4 Richard Harris, “Heroin Use Surges, Especially among Women and Whites,” NPR, July 7, 2015, http://www.npr.org/sections/health-shots/2015/07/07/420874860/heroin-use-surges-especially-among-women-and-whites.
5 Theodore J. Cicero, Matthew S. Ellis, and Zachary A. Kasper, “Increased Use of Heroin as an Initiating Opioid of Abuse,” Addictive Behaviors 74 (November 2017): 63–66, doi.org/10.1016/j.addbeh.2017.05.030.
6 Drug Policy Alliance, “Harm Reduction,” http://www.drugpolicy.org/issues/harm-reduction.
7 Bob Young, “Initiative Proposed to Ban Heroin Safe-Injection Sites in King County,” Seattle Times, May 11, 2017.
8 Anna Giaritelli, “Seattle Could Be First City to Give Heroin Users ‘Safe Spaces,’” Washington Examiner, May 15, 2017.
9 Carlos Nordt and Rudolf Stohler, “Incidence of Heroin Use in Zurich, Switzerland: A Treatment Case Register Analysis,” Lancet 367, no. 9525 (June 2006): 1830–34.
10 Jill Gonzalez, “Treating Opiate Addiction with Replacement Therapy,” CRC Health, http://www.crchealth.com/find-a-treatment-center/opiate-addiction-treatment/oxycontin-articles/treating-opiate-addiction-replacement-therapy/.
11 Jake Harper, “Price’s Remarks on Opioid Treatment Were Unscientific and Damaging, Experts Say,” NPR, May 16, 2017, https://www.npr.org/sections/health-shots/2017/05/16/528614422/prices-remarks-on-opioid-treatment-were-unscientific-and-damaging-experts-say.
12 Kate Sheridan, “How Effective Is Medication-Assisted Treatment for Addiction? Here’s the Science,” STAT, May 15, 2017, https://www.statnews.com/2017/05/15/medication-assisted-treatment-what-we-know/; and National Institute on Drug Abuse, “Effective Treatments for Opioid Addiction,” last updated November 2016, https://www.drugabuse.gov/publications/effective-treatments-opioid-addiction/effective-treatments-opioid-addiction.
13 For more on abuse-deterrent formulations of opioids and evergreening, see Jeffrey A. Singer, “Abuse-Deterrent Opioids and the Law of Unintended Consequences,” Cato Institute Policy Analysis no. 832, February 6, 2018, https://object.cato.org/sites/cato.org/files/pubs/pdf/pa832.pdf.
14 Paul Alexander, “As Manufacturer of Leading Addiction Drug Comes under Legal Fire, a New Competitor Emerges,” Huffington Post, October 27, 2016, https://www.huffingtonpost.com/entry/as-manufacturer-of-leading-addiction-drug-comes-under_us_5811abb4e4b08301d33e058f.
15 Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act, 115 Pub. L. No. 271, 132 Stat. 3894 (2018), https://www.congress.gov/bill/115th-congress/house-bill/6/text#toc-H626C793620EE42D89660E8AB85724CCF. See generally, American Society of Addiction Medicine, “Nurse Practitioners and Physician Assistants Prescribing Buprenorphine,” https://www.asam.org/resources/practice-resources/nurse-practitioners-and-physician-assistants-prescribing-buprenorphine.
16 Stewart B. Leavitt, “A Community-Centered Solution for Opioid Addiction: Methadone Maintenance Treatment (MMT),” Addiction Treatment Forum, May 2004, http://atforum.com/documents/com_ctrd_mmt.pdf.
17 Robert E. Booth, Karen F. Corsi, and Susan K. Mikulich-Gilbertson, “Factors Associated with Methadone Maintenance Treatment Retention among Street-Recruited Injection Drug Users,” Drug and Alcohol Dependence 74, no. 2 (May 10, 2004): 177–85, https://doi.org/10.1016/j.drugalcdep.2003.12.009.
18 Einat Peles et al., “One-Year and Cumulative Retention as Predictors of Success in Methadone Maintenance Treatment: A Comparison of Two Clinics in the United States and Israel,” Journal of Addictive Diseases 27, no. 4 (2008): 11–25, https://doi.org/10.1080/10550880802324382.
19 Dennis Deck and Matthew J. Carlson, “Retention in Publicly Funded Methadone Maintenance Treatment in Two Western States,” Journal of Behavioral Health Services and Research 32, no. 1 (January 2005): 43–60, https://doi.org/10.1007/BF02287327.
20 Luis Sordo et al., “Mortality Risk during and after Opioid Substitution Treatment: Systematic Review and Meta-analysis of Cohort Studies,” BMJ 357 (April 26, 2017): j1550, https://doi.org/10.1136/bmj.j1550.
21 Richard P. Mattick et al., “Buprenorphine Maintenance vs. Placebo or Methadone Maintenance for Opioid Dependence,” Cochrane Database of Systematic Reviews no. 2 (2014), https://www.ncbi.nlm.nih.gov/pubmed/24500948.
22 Paul J. Whelan and Kimberly Remski, “Buprenorphine vs. Methadone Treatment: A Review of Evidence in Both Developed and Developing Worlds,” Journal of Neurosciences in Rural Practice 3, no. 1 (2012): 45–50, https://doi.org/10.4103/0976–3147.91934.
23 Adam N. Peddicord, Chris Bush, and Crystal Cruze, “A Comparison of Suboxone and Methadone in the Treatment of Opiate Addiction,” Journal of Addiction Research and Therapy 6 (November 27, 2015): 248, https://doi.org/10.4172/2155–6105.1000248.
24 Silvia Minozzi et al., “Oral Naltrexone Maintenance Treatment for Opioid Dependence,” Cochrane Database of Systematic Reviews no. 4 (April 13, 2011), https://doi.org/10.1002/14651858.CD001333.pub4.
25 Gavin Bart, “Maintenance Medication for Opiate Addiction: The Foundation of Recovery,” Journal of Addictive Diseases 31, no. 3 (July 2012): 2017-225, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3411273/.
26 Jeffrey A. Singer, “Methadone and Mixed Messages,” Cato at Liberty (blog entry), July 13, 2018, https://www.cato.org/blog/methadone-mixed-messages.
27 “Drug and Alcohol Rehab in Tacoma, WA,” DrugRehab.com, https://www.drugrehab.com/washington/tacoma/.
28 Traci C. Green et al., “Life after the Ban: An Assessment of U.S. Syringe Exchange Programs’ Attitudes about and Early Experiences with Federal Funding,” American Journal of Public Health 102, no. 5 (May 1, 2012): e9–e16, https://ajph.aphapublications.org/doi/10.2105/AJPH.2011.300595.
29 Don C. Des Jarlais et al., “Doing Harm Reduction Better: Syringe Exchange in the United States,” Addiction 104 (2009): 1441–46, http://citeseerx.ist.psu.edu/viewdoc/download;jsessionid=3C228AE41B62553018D38E76D37A3B88?doi=10.1.1.687.6190&rep=rep1&type=pdf.
30 “Federal Research on Syringe Exchange Programs Proves Effectiveness,” Drug War Facts, http://www.drugwarfacts.org/node/924.
31 Abu S. Abdul-Quader et al., “Effectiveness of Structural-Level Needle/Syringe Programs to Reduce HCV and HIV Infection among People Who Inject Drugs: A Systematic Review,” AIDS and Behavior 17 (2013): 2878–92, doi.org/10.1007/s10461-013‑0593‑y.
32 Esther J. Aspinall et al., “Are Needle and Syringe Programmes Associated with a Reduction in HIV Transmission among People Who Inject Drugs? A Systematic Review and Meta-analysis,” International Journal of Epidemiology 43, no. 1 (February 2014): 235–48, https://doi.org/10.1093/ije/dyt243.
33 Substance Abuse and Mental Health Services Administration, “Syringe Exchange Program Studies,” last updated February 24, 2011, https://web.archive.org/web/20161223195811/http:/archive.samhsa.gov/ssp/.
34 CDC, “Syringe Services Programs,” last updated April 19, 2018, https://www.cdc.gov/hiv/risk/ssps.html.
35 Mikel Chavers, “Swapping Needles: States Fight an Epidemic with Clean Syringes,” Council of State Governments, May 2008, http://www.csg.org/knowledgecenter/docs/sn0805SwappingNeedles.pdf; Matt Smith, “Move to Allow Needle Exchange Programs without State Approval Awaits Gov. Holcomb’s Signature,” Fox59-WXIN, Indianapolis, April 10, 2017; and Leo Beletsky et al., “Police Encounters among Needle Exchange Clients in Baltimore: Drug Law Enforcement as a Structural Determinant of Health,” American Journal of Public Health 105, no. 9 (September 2015): 1872–79, https://www.ncbi.nlm.nih.gov/pubmed/26180948.
36 Leo Beletsky, Grace Macalinom, and Scott Burris, “Attitudes of Police Officers towards Syringe Access, Occupational Needle-Sticks, and Drug Use: A Qualitative Study of One City Police Department in the United States,” International Journal of Drug Policy 16 (2005): 267, https://ssrn.com/abstract=872756.
37 Leo Beletsky et al., “The Roles of Law, Client Race and Program Visibility in Shaping Police Interference with the Operation of US Syringe Exchange Programs,” Addiction 106 (November 4, 2010): 357–65, https://doi.org/10.1111/j.1360–0443.2010.03149.x.
38 City of Vancouver, “Safe Injection Site and Needle Exchange,” http://vancouver.ca/people-programs/safe-injection-site-and-needle-exchange.aspx.
39 Drug Policy Alliance, “Supervised Injection Facilities,” February 2016, https://www.drugpolicy.org/sites/default/files/DPA%20Fact%20Sheet_Supervised%20Injection%20Facilities%20%28Feb.%202016%29.pdf.
40 Kate Dolan et al., “Drug Consumption Facilities in Europe and the Establishment of Supervised Injecting Centres in Australia,” Drug and Alcohol Review 19, no. 3 (2000): 337–46, http://www.tandfonline.com/doi/abs/10.1080/cdar.19.3.337.346?src=recsys&.
41 Tim Rhodes and Dagmar Hedrich, eds., Harm Reduction: Evidence, Impacts, and Challenges, EMCDDA Monograph no. 10 (Luxembourg: European Monitoring Centre for Drugs and Drug Addiction, 2010), http://www.emcdda.europa.eu/system/files/publications/555/EMCDDA-monograph10-harm_reduction_final_205049.pdf_en.
42 Drug Policy Alliance, “Supervised Injection Facilities.”
43 Kate Dolan and Alex Woodak, “Final Report on Injecting Rooms in Switzerland,” BurgerForeningen, April 4, 2013, http://brugerforeningen.dk/2013/04/final-report-on-injecting-rooms-in-switzerland/?lang=en.
44 British Columbia Centre on Substance Use, “Study: Supervised Injection Site Reduces HIV Risk Behaviour,” March 17, 2005, https://www.bccsu.ca/news-release/study-supervised-injection-site-reduces-hiv-risk-behaviour/.
45 Urban Health Research Initiative, “Insight into Insite,” January 15, 2010, http://www.cfenet.ubc.ca/sites/default/files/uploads/publications/insight_into_insite.pdf.
46 “Drug Consumption Rooms in Germany: A Situational Assessment by the AK Konsumraum,” Deutsche AIDS-Hilfe and Azkept, http://www.akzept.org/pdf/aktuel_pdf/DKR07af1Eng.pdf.
47 Carrie A. Lingle, “A Critical Review of the Effectiveness of Safe Injection Facilities as a Harm Reduction Strategy,” University of Pittsburgh Graduate School of Public Health, April 9, 2013, http://d‑scholarship.pitt.edu/18375/1/CarrieLingle_-_FinalThesisEssay.pdf.
48 Brandon D. L. Marshall et al., “Reduction in Overdose Mortality after the Opening of North America’s First Medically Supervised Safer Injecting Facility: A Retrospective Population-Based Study,” Lancet 377, no. 9775 (April 23, 2011): 1429–37, https://doi.org/10.1016/S0140-6736(10)62353–7.
49 Benjamin Preiss and Josh Gordon, “Support Growing for Safe Injecting Room in Melbourne,” Brisbane Times, February 6, 2017, https://www.brisbanetimes.com.au/national/victoria/support-growing-for-safe-injecting-room-in-melbourne-20170206-gu6r3x.html; and Alcohol and Drug Foundation, “Medically Supervised Injecting Centres,” February 17, 2017, https://adf.org.au/insights/medically-supervised-injecting-centres/.
50 CDC, “Syringe Services Programs,” last updated April 19, 2018, https://www.cdc.gov/hiv/risk/ssps.html.
51 Chris Elkins, “Update: Seattle Approves Safe Sites for Drug Use,” DrugRehab.com, September 16, 2016, https://www.drugrehab.com/2016/09/16/safe-drug-consumption-sites-in-seattle/; and David Gutman, “Safe Heroin Injection Sites Get OK from King County Health Board,” Seattle Times, January 19, 2017.
52 Peter Johnson, “Washington Using Needle Exchanges, Injection Sites to Combat Addiction,” DrugRehab.com, April 17, 2017, https://www.drugrehab.com/2017/04/17/washington-needle-exchanges-and-safe-injection-sites/.
53 Dominic Fracassa, “Strong Support for Safe Injection Centers in SF,” San Francisco Chronicle, August 10, 2017.
54 Maia Szalavitz, “There’s Been a Secret Safe Injection Site in the US for Three Years,” Tonic, August 10, 2017, https://tonic.vice.com/en_us/article/433ynj/theres-been-a-secret-safe-drug-injection-site-in-the-us-for-three-years.
55 Alex H. Kral and Peter J. Davidson, “Addressing the Nation’s Opioid Epidemic: Lessons from an Unsanctioned Supervised Injection Site in the U.S.,” American Journal of Preventive Medicine 53, no. 6 (December 2017): 919–22, https://doi.org/10.1016/j.amepre.2017.06.010.
56 Wendy Stueck, “The Arguments for and against Vancouver’s Supervised Injection Site,” Globe and Mail, May 11, 2011.
57 Nadia Whitehead, “How the Prescription Painkiller Fentanyl Became a Street Drug,” NPR, August 26, 2015.
58 Josh Sanburn, “Heroin Is Being Laced with a Terrifying New Substance: What to Know about Carfentanil,” Time, September 12, 2016.
59 Christian Haasen et al., “Heroin-Assisted Treatment for Opioid Dependence: Randomised Controlled Trial,” British Journal of Psychiatry 191, no. 1 (July 2007): 55–62, https://www.ncbi.nlm.nih.gov/pubmed/17602126.
60 Garry V. Stimson and Nicky Metrebian, Prescribing Heroin: What Is the Evidence? (York, UK: Joseph Roundtree Foundation, 2003), https://www.jrf.org.uk/sites/default/files/jrf/migrated/files/1859350836.pdf.
61 Nordt and Stohler, “Incidence of Heroin Use in Zurich, Switzerland.”
62 Marica Ferri, Marina Davoili, and Carlo A. Perucci, “Heroin Maintenance for Chronic Heroin-Dependent Individuals” Cochrane Database of Systematic Reviews no. 12 (2011), https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD003410.pub4/information.
63 Howard J. Wooldridge, “Swiss Medication-Assisted Treatment 1994–2018: Summary,” Citizens Opposing Prohibition, http://www.citizensopposingprohibition.org/resources/swiss-heroin-assisted-treatment-1994–2009-summary/; and Nordt and Stohler, “Incidence of Heroin Use in Zurich, Switzerland.”
64 Urs Geiser, “Swiss to Agree Heroin Scheme but Say No to Dope,” Swissinfo.ch, November 30, 2008, http://www.swissinfo.ch/eng/swiss-to-agree-heroin-scheme-but-say-no-to-dope/7071120.
65 Uwe Verthein et al., “Long-Term Effects of Heroin-Assisted Treatment in Germany,” Addiction 103 (2008): 960–66, https://doi.org/10.1111/j.1360–0443.2008.02185.x.
66 Peter Blanken et al., “Outcome of Long-Term Heroin-Assisted Treatment Offered to Chronic, Treatment-Resistant Heroin Addicts in the Netherlands,” Addiction 105 (2010): 300–308, https://doi.org/10.1111/j.1360–0443.2009.02754.x.
67 Uwe Verthein, Silke Kuhn, and Christian Haasen, “Das Bundesdeutsche Modellprojekt zur Heroingestützten Behandlung Opiatabhängiger—Eine Multizentrische, Randomisierte, Kontrollierte Therapiestudie,” Zentrum für Interdisziplinäre Suchtforschung der Universität Hamburg, Germany, January 2008, http://www.heroinstudie.de/H‑Bericht_FU.pdf.
68 Canadian Press, “Canada Now Allows Prescription Heroin in Severe Opioid Addiction,” CBC, September 8, 2016.
69 “Heroin Assisted Treatment (HAT): Saving Lives, Improving Health, Reducing Crime,” Transform: Getting Drugs under Control (blog), March 29, 2017, http://www.tdpf.org.uk/blog/heroin-assisted-treatment-hat-saving-lives-improving-health-reducing-crime.
70 John Strang, Teodora Groshkova, and Nicola Metrebian, “New Heroin-Assisted Treatment: Recent Evidence and Current Practices of Supervised Injectable Heroin Treatment in Europe and Beyond,” EMCDDA Insights no. 11, European Monitoring Centre for Drugs and Drug Addiction, 2012, http://www.emcdda.europa.eu/attachements.cfm/att_154996_EN_Heroin percent20Insight.pdf.
71 Eugenia Oviedo-Joekes et al., “Diacetylmorphine vs. Methadone for the Treatment of Opioid Addiction,” New England Journal of Medicine 361 (August 20, 2009): 777–86, https://doi.org/10.1056/NEJMoa0810635.
72 Kirsten I. Marchand et al., “Client Satisfaction among Participants in a Randomized Trial Comparing Oral Methadone and Injectable Diacetylmorphine for Long-Term Opioid-Dependency,” BMC Health Services Research 11 (July 26, 2011): 174, https://doi.org/10.1186/1472–6963-11–174.
73 Bohdan Nosyk et al., “Cost-Effectiveness of Diacetylmorphine vs. Methadone for Chronic Opioid Dependence Refractory to Treatment,” Canadian Medical Association Journal 184, no. 6 (March 12, 2012): E317–E328, https://doi.org/10.1503/cmaj.110669.
74 “Opioid Analgesics: Approximate Potency Equivalence with Oral Morphine,” Monthly Index of Medical Specialties, Nov. 25, 2015, https://www.mims.co.uk/opioid-analgesics-approximate-potency-equivalence-oral-morphine/pain/article/1146201; and Richard Stockton, “Dangerous Street Drugs That Your Doctor Can Totally Prescribe Right Now,” ATI, May 4, 2018, http://allthatsinteresting.com/medical-heroin-cocaine; Newsbeat, “The Illegal Drugs with Legal Medical Uses,” BBC, April 6, 2017.
75 CDC, “Expanding Naloxone Use Could Reduce Drug Overdose Deaths and Save Lives,” press release, CDC, April 24, 2015, https://www.cdc.gov/media/releases/2015/p0424-naloxone.html.
76 Karen Mahoney, “FDA Supports Greater Access to Naloxone to Help Reduce Opioid Overdose Deaths,” FDA Voices (blog), August 10, 2016, https://www.pharmacist.com/article/fda-supports-greater-access-naloxone-help-reduce-opioid-overdose-deaths.
77 “Treatment of Opioid Dependence,” World Health Organization, http://www.who.int/substance_abuse/activities/treatment_opioid_dependence/en/.
78 Corey S. Davis et al., “Expanded Access to Naloxone among Firefighters, Police Officers, and Emergency Medical Technicians in Massachusetts,” American Journal of Public Health 104, no. 8 (August 1, 2014): e7–e9, https://doi.org/10.2105/AJPH.2014.302062.
79 Rebecca E. Giglio, Guohua Li, and Charles J. DiMaggio, “Effectiveness of Bystander Naloxone Administration and Overdose Education Programs: A Meta-analysis,” Injury Epidemiology 2, no. 1 (December 2015): 10, https://dx.doi.org/10.1186%2Fs40621-015‑0041‑8.
80 “Naloxone Education and Distribution Programs,” County Health Rankings and Roadmaps, last updated February 24, 2017, http://www.countyhealthrankings.org/policies/naloxone-education-distribution-programs.
81 Courtney Columbus, Cronkite News, “Naloxone: Opioid Overdose Reversal Drug in Arizona,” Prescott eNews, March 7, 2017, http://www.prescottenews.com/index.php/news/current-news/item/29537-naloxone-opioid-overdose-reversal-drug-in-arizona.
82 Network for Public Health Law, “Legal Interventions to Reduce Overdose Mortality: Naloxone Access and Overdose Good Samaritan Laws,” https://www.networkforphl.org/_asset/qz5pvn/network-naloxone-10–4.pdf.
83 Caleb J. Banta-Green et al., “Washington’s 911 Good Samaritan Drug Overdose Law—Initial Evaluation Results,” Alcohol and Drug Abuse Institute, University of Washington, November 2011, http://adai.uw.edu/pubs/infobriefs/ADAI-IB-2011–05.pdf.
84 Daniel Rees et al., “With a Little Help from My Friends: The Effects of Naloxone Access and Good Samaritan Laws on Opioid-Related Deaths,” Cato Research Briefs in Economic Policy no. 78, June 2017, https://object.cato.org/sites/cato.org/files/pubs/pdf/research-brief-78.pdf.
85 “Naloxone: Frequently Asked Questions,” NaloxoneInfo.org, http://naloxoneinfo.org/sites/default/files/Frequently%20Asked%20Questions-Naloxone_EN.pdf.
86 Jeffrey A. Singer, “To Save Lives, Make Naloxone an Over-the-Counter Drug,” Reason, April 27, 2018, https://reason.com/archives/2018/04/27/to-combat-opioid-abuse-the-surgeon-gener.
87 “State Marijuana Laws in 2018 Map,” Governing, March 30, 2018, http://www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html.
88 “State Medical Marijuana Laws Linked to Lower Prescription Overdose Deaths,” news release, Johns Hopkins Bloomberg School of Public Health, August 26, 2014, https://www.jhsph.edu/news/news-releases/2014/state-medical-marijuana-laws-linked-to-lower-prescription-overdose-deaths.html.
89 David Powell, Rosalie Liccardo Pacula, and Mireille Jacobson, “Do Medical Marijuana Laws Reduce Addictions and Deaths Related to Pain Killers?” Journal of Health Economics 58 (March 2018): 29–42, https://doi.org/10.1016/j.jhealeco.2017.12.007.
90 Laurel Thomas, “Medical Marijuana Reduces Use of Opioid Pain Meds, Decreases Risk for Some with Chronic Pain,” Michigan News, March 22, 2016, http://ns.umich.edu/new/releases/23622-medical-marijuana-reduces-use-of-opioid-pain-meds-decreases-risk-for-some-with-chronic-pain.
91 Amanda Reiman, Mark Welty, and Perry Solomon, “Cannabis as a Substitute for Opioid-Based Pain Medication: Patient Self-Report,” Cannabis and Cannabinoid Research 2, no. 1 (June 1, 2017), https://doi.org/10.1089/can.2017.0012.
92 Ashley C. Bradford et al., “Association between U.S. State Medical Cannabis Laws and Opioid Prescribing in the Medicare Part D Population,” JAMA Internal Medicine 178, no. 5 (2018): 667–72, https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/2676999.
93 Hefei Wen and Jason M. Hockenberry, “Association of Medical and Adult-Use Marijuana Laws with Opioid Prescribing for Medicaid Enrollees,” JAMA Internal Medicine 178, no. 5 (2018): 673–79, https://jamanetwork.com/journals/jamainternalmedicine/article-abstract/2677000?redirect=true.
94 David P. Wilson et al., “The Cost-Effectiveness of Harm Reduction,” International Journal of Drug Policy 26 suppl. 1 (February 2015): S5–S11, https://doi.org/10.1016/j.drugpo.2014.11.007.
95 Ehsan Jozaghi, “Exploring the Role of an Unsanctioned, Supervised Peer Driven Injection Facility in Reducing HIV and Hepatitis C Infections in People that Require Assistance during Injection,” Health and Justice 3, no. 16 (August 2015), https://doi.org/10.1186/s40352-015‑0028‑0.
96 Herlianna Naning et al., Return on Investment and Cost-Effectiveness of Harm Reduction Program in Malaysia, (Washington: World Bank Group, 2014), http://hdl.handle.net/10986/18641.
97 West Virginia Department of Health and Human Resources, Bureau of Public Health, “The Need for Harm Reduction Programs in West Virginia,” white paper, November 6, 2017, https://dhhr.wv.gov/oeps/harm-reduction/Documents/HRP%20White%20Paper-FINAL.pdf.
98 Dylan Scott, “The Harrowing Rise of Heroin, in One Chart,” Vox, October 5, 2017, https://www.vox.com/policy-and-politics/2017/10/5/16432418/voxcare-rise-of-heroin-one-chart.
99 Jeffrey A. Singer, “The Myth of an Opioid Prescription Crisis,” Cato Policy Report, September/October 2017, https://www.cato.org/policy-report/septemberoctober-2017/myth-opioid-prescription-crisis; and Jeffrey A. Singer, “Misdiagnosing the Opioid Crisis,” Inside Sources, September 27, 2017, also available at https://www.cato.org/publications/commentary/misdiagnosing-opioid-crisis.
100 Jeffrey A. Singer, “Let’s Stop the Hysterical Rhetoric about the Opioid Crisis,” Townhall.com, August 31, 2017, also available at https://www.cato.org/publications/commentary/lets-stop-hysterical-rhetoric-about-opioid-crisis.
101 Maia Szalavitz, “Opioid Addiction Is a Huge Problem, but Pain Prescriptions Are Not the Cause,” Scientific American (MIND Guest blog), May 10, 2016, https://blogs.scientificamerican.com/mind-guest-blog/opioid-addiction-is-a-huge-problem-but-pain-prescriptions-are-not-the-cause/.
102 Christopher Moraff, “Feds’ Pill Crackdown Drives Pain Patients to Heroin,” Daily Beast, April 15, 2016.
103 Lydia Ramsey, “‘We’re Treated Like Drug Addicts’: As America Fights Opioid Addiction, the Healthcare System Is Failing People Who Live with Chronic Pain,” Business Insider, January 28, 2018; and Robert Langreth, “Millions of Patients Face Pain and Withdrawal as Opioid Prescriptions Plummet,” Bloomberg Business, November 21, 2017.
104 Thomas Kline, “#OpioidCrisis Pain Related Suicides Associated with Forced Tapers,” Medium, May 30, 2018.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.